Investigating the mechanisms of and overcoming cell-mediated immunosuppression of CAR T cells, using in vitro multicellular models
Primary supervisor: John Marshall, Queen Mary University of London
Secondary supervisor: Adrian Biddle, Queen Mary University of London
Collaborator: James Arnold, King’s College London
Project
Chimeric Antigen Receptor (CAR) T cell therapy has delivered a major breakthrough in the effective therapy of several blood cancers. CAR T therapy re-engineers T cells to express an additional receptor directed to a cancer-specific antigen and infusing them into the cancer bearing host has dramatically improved survival from, particularly, B-cell derived cancer [1]. However, over 90% of cancer develops as solid tumours in tissues and CAR T cell therapy has failed to have even limited success. Reasons cited include fibrous collagen barriers laid down by activated fibroblasts (CAFs), pro-tumourigenic and immunosuppressive cytokines of CAFs [1] in the tumour microenvironment (TME) and the strong immunosuppressive signalling (including TGFb) that switches off innate and immune cytotoxic cells, including CAR T cells.
To improve CAR T therapy of human disease we need to dissect the molecular response of all the relevant cells (cancer, fibroblasts/CAFs, CAR T, macrophages (Type 1/Type2), TReg, endothelial cells- all generated in vitro) at the site of action. Using 3D organotypic gels and tumour-on-chip microfluidic systems we will challenge synthetic tumours (cancer cells, fibroblasts, endothelial cells) with CAR T and NK-CAR T, in the presence and absence of type 1 and type 2 macrophages and TReg cells. Monitoring cell-cell interactions and molecular behaviours will be measured using fluorescent dyes. Combining ‘Biotracker’ fluorescent dye-labelling with fluorescent reporters of actin cytoskeletal changes (Lifeact), mechano-transduction activity (from Dr Angus Cameron, BCI), collagen production and deposition patterns, TGFb signalling, lysosome (Lysotracker) we can monitor these pathways and the location of every cell type in the culture and measure live using Imaris XT 3D software
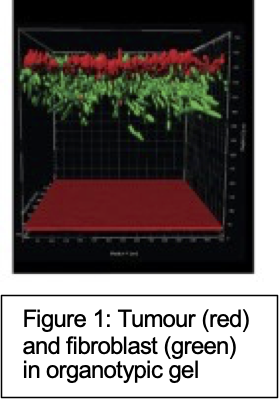
- Number, motility and location of effectors
- Number of effector:cancer cell events
- Number and location of lysosomes (location of granzyme and perforin) in effectors
- Number of effector:cancer cell events that lead to cancer cell death
- Health of effectors and targets
- Behaviour of fibroblasts/CAFs
Gels will be processed for antibody analysis of exhaustion markers of effectors. We will establish the baseline data of how effectors behave adjacent to a ‘tumour’. We shall use CARs against integrin avb6 as these have shown efficacy in vitro and in vivo [2]. Following our study delivering IL12 and anti-TGFb improves CAR T penetration of solid tumours [3] we will introduce compounds to target TReg (anti-avb8) or immunosuppressive signals (anti-TGFb, CXR4, CXCL12, PD1, PDL-1) at different timepoints, assess each cell type but also if effector motility and killing is improved. We can generate detailed 3D images of organotypics (Figure 1a) using spinning disk confocal microscopy capturing up to 7 colours.
The organotypic gel assays will identify the key cell types limiting effector activity. Key assays will be replicated in tumour-on-chip assays that have already been developed [4] for InCell6000 confocal scanner. Endothelial and cancer-fibroblast/CAF mixtures are established before immune suppressive cells (macrophages, Tregs) and effectors are added, the same as the organotypic model. The tumour-on-chip will be adaptable for medium/high throughput screening for novel CAR T strategies.
References
- Cutmore LC, Brown NF, Raj D, Chauduri S, Wang P, Maher J1, Wang Y, Lemoine NR, Marshall JF. Pancreatic Cancer UK Grand Challenge: Developments and challenges for effective CAR T cell therapy for pancreatic ductal adenocarcinoma Pancreatology 2020 20:394-408 Review
- Whilding LM, Parente-Pereira AC, Zabinski T, Davies DM, Petrovic RMG, Kao YV, Saxena SA, Romain A, Costa-Guerra JA, Violette S, Itamochi H, Ghaem-Maghami S, Vallath S, Marshall JF, Maher J. Targeting of Aberrant αvβ6 Integrin Expression in Solid Tumors Using Chimeric Antigen Receptor-Engineered T Cells. Mol Ther. 2017 Jan 4;25(1):259-273.
- The Combination of Oncolytic Virus and Antibody Blockade of TGF-β Enhances the Efficacy of αvβ6-Targeting CAR T Cells Against Pancreatic Cancer in an Immunocompetent Model. Zuoyi Zhao , Lauren C. Cutmore , Renato B. Baleeiro , Joseph J. Hartlebury , Nicholas Brown, Louisa Chard-Dunmall , Nicholas Lemoine, Yaohe Wang * and John F. Marshall *. Cancers 2025, 17, 1534
- Scemama A, Lunetto S, Tailor A, Di Cio S, Dibble M, Gautrot J, Biddle A ( 2024 ) .Development of an in vitro microfluidic model to study the role of microenvironmental cells in oral cancer metastasis .12 , 10.12688/f1000research.131810.2
- Deepak Raj1, Maria Nikolaidi1, Irene Garces1, Daniela Lorizio1, Natalia M. Castro1, Sabrina G. Caiafa1, Kate Moore1, Nicholas F. Brown1, Hemant M. Kocher2, Xiaobo Duan3,4, Brad H. Nelson3,4, Nicholas R. Lemoine1 and John F. Marshall. CEACAM7 is an effective target for CAR-T cell therapy of pancreatic ductal adenocarcinoma Clin Cancer Res 2021 27(5):1538-1552.
