Development of allogeneic Natural Killer (NK) cells transduced with Chimeric Antigen Receptors (CARs) for paediatric and adult Acute Myeloid Leukaemia (AML) and high risk myelodysplasia (MDS)
Primary supervisor: Sara Ghorashian, UCL
Secondary supervisor: Shahram Kordasti, King’s College London
Project
Background:
We have developed chimeric antigen receptors (CARs) specific for the AML antigens CD123, CD33 and CLL1 (CLEC12A) derived from single domain binders (ref 1). Currently these are being investigated for translation with autologous T cells as the effector cell population. However, such products have to be created on a bespoke, per-patient basis. For rapidly progressive acute leukaemias such as advanced relapsed AML, there may not be a window to harvest autologous T cells from the patient and await a minimum of 4 weeks for CAR T cell product to be generated. Further, the feasibility of manufacture may be impacted by prior therapy e.g. cytarabine given in re-induction.
We have therefore explored use of allogeneic NK cells as a CAR effector population as allogeneic NK cells can be infused without risk of GVHD and unmanipulated NK have been trialled as a therapy for AML for many years (ref 2). Further, this approach has been successful in CD19 CAR T cell targeting of B cell haematological malignancies (ref 3).
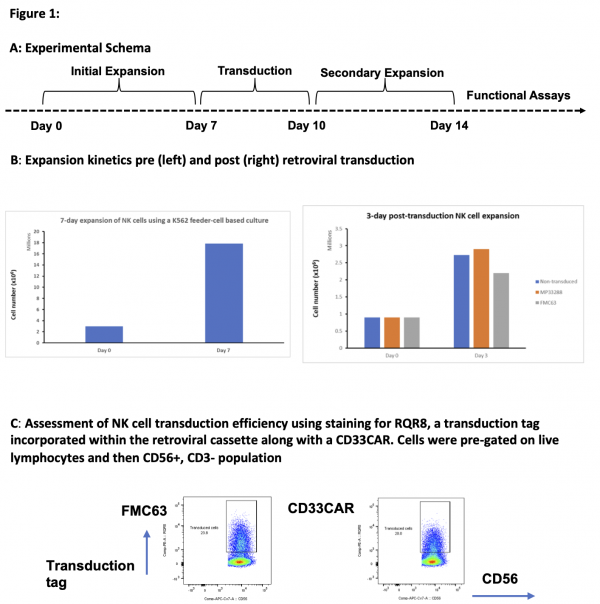
We have expanded NK cells isolated from cord blood and successfully transduced them using retroviral vectors to express CD33 CAR (figure 1)
Aims/Objectives:
To develop optimised CAR NK cells for AML therapy:
- To investigate the optimal source of NK cells for AML therapy (umbilical cord blood, peripheral blood from a healthy adult donor or induced pleuripotent stem cell (iPSC) – derived
- To develop optimised lenti or retroviral constructs for NK cell CAR transduction with single or multiple AML CARs
- To develop optimal transduction protocols for generating CAR NK cells from these sources
- To develop CAR NK cells with additional modifications e.g. IL-15 secretion/signalling / other cytokine expression / signalling moieties to improve anti-tumour efficacy, memory function and persistence
Methods:
- CAR NK cells will be generated from a variety of sources. Their antigen-specific responses (cytotoxicity, proliferation, cytokine production) against target cells expressing AML antigens will be compared in single and repetitive stimulations with antigen-bearing target cells
- Optimised lenti or retroviral constructs for NK cell CAR transduction with single or multiple AML CARs will be cloned and tested. Baboon enveloped pseudotyped lentiviral constructs have been shown to provide high efficiency transduction (ref 4)
- Optimal transduction protocols for generating CAR NK cells will be tested to maximise transduction efficiencies and CAR NK cell yield
- CAR NK cells with additional modifications e.g. IL-15 / other cytokine expression / signalling moieties to improve anti-tumour efficacy and persistence (ref 5) will be generated and tested in vitro (as in 1, in repetitive stimulation assays to assess exhaustion/memory potential) as well as in relevant in vivo models. These include e.g. immunodeficient (NSG) mice bearing established MOLM14ffluc or patient-derived AML tumours. The latter animal models have been validated and established in our lab
References
- Hazelton (PhD student) et al., Nanobody Based Tri-Specific Chimeric Antigen Receptor to Treat Acute Myeloid Leukaemia. Blood 2020; 136 (Supplement 1): 10-11 [Link]
- Rubnitz et al., Natural killer cell therapy in children with relapsed leukemia, Pediatric Blood & Cancer, vol. 62, no. 8, pp. 1468-1472, 2015.
- Liu et al., Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. NEJM 2020 DOI: 10.1056/NEJMoa1910607
- Colamartino et al., Efficient and Robust NK-Cell Transduction With Baboon Envelope Pseudotyped Lentivector. Front Immunol. 2019 Dec 16;10:2873. doi: 10.3389/fimmu.2019.02873
- Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022 Oct;22(10):557-575. doi: 10.1038/s41568-022-00491-0. Epub 2022 Jul 25
