Theragnostic Nanoparticles Core Facility
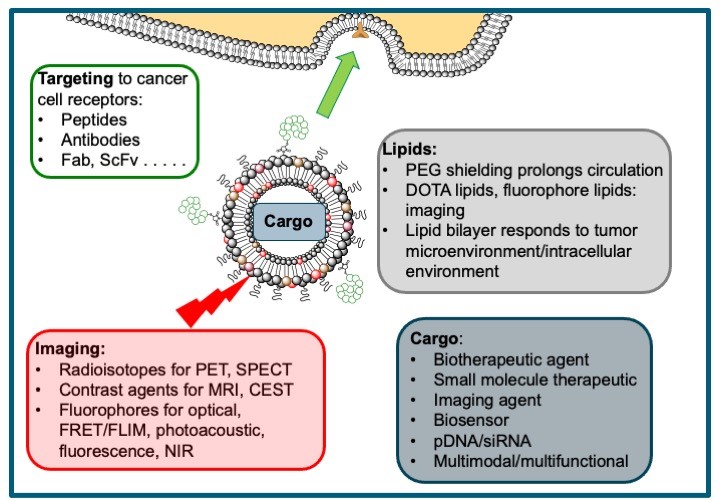
The Theragnostic Nanoparticles Core Facility is based at UCL Chemistry. We are developing targeted, liposome-based theragnostic nanoparticles from cancer imaging and therapeutic delivery. Using a toolbox of self-assembling lipid and peptide components we can formulate nanoparticles that can deliver a variety of cargoes – small molecule therapeutics, biotherapeutics, plasmid DNA, siRNA – and selectively deliver these to tumours using peptide or antibody targeting to receptors overexpressed on cancer cells. Liposomal encapsulation of the cargo in nanoparticles 100-150 nm, and a combination of targeting and the EPR effect, allows patients to be shielded from off-target effects of the therapeutic cargo. The modular nature of these liposome-based nanoparticles means that we can also incorporate a variety of tracers for PET, SPECT, MRI, optical or photoacoustic imaging.
We welcome collaborations from groups working across all cancer cell types and imaging modalities: please contact us to discuss further.
Contact
Academic Lead
Facilities and Equipment
As well as expertise and equipment for standard organic synthesis, our core has extensive expertise and facilities for solid-phase peptide synthesis (Syro II and Biotage Initiator + Alstra peptide synthesisers), peptide bioconjugation and purification (analytical and preparative HPLC). The Department of Chemistry is well equipped with a suite of 5 high field NMRs including Brucker 700 MHz broadband cryoprobe and spectrometer) and extensive mass spectrometry facilities.
Formulation of homogeneous populations of liposome-based nanoparticles is carried out by extrusion (LIPEX extruder) and size, polydispersity and surface charge are routinely measured (Malvern Zetasizer). Standard TEM facilities are available at the Department of Chemistry, and we also collaborate with groups at KCL, UCL and the London Centre for Nanotechnology for cryoTEM, liquid TEM and AFM experiments.
What sort of nanoparticles can we make?
Liposomal formulations of anticancer drugs such as doxorubicin are beginning to enter clinical use as delivery systems that reduce the toxicity of these drugs to non-cancerous cells. By tuning the lipid composition, for instance by including PEG and n-ethylene glycol (n-EG) lipids to prolong systemic circulation, and by including DOTA-lipids or fluorophore-modified lipids, we can prepare liposomes that can be used for multimodal imaging as well as delivery of small molecule therapeutics and biotherapeutics. Liposomal encapsulation of contrast agents is also a powerful tool for improving sensitivity and selectivity, and we have recently demonstrated excellent contrast and sensitivities of LipoGlucoCEST and Lipo2DG-CEST formulations.
Demetriou, E., Story, H., Bofinger, R., Hailes, H. C., Tabor, A. B., Golay, X. (2019) The effect of liposomal encapsulation on the chemical exchange properties of diamagnetic CEST agents. J Phys Chem B, 123, 7545 – 7557. https://pubs.acs.org/doi/10.1021/acs.jpcb.9b02280
Mitchell, N., Kalber, T. L., Cooper, M. S., Sunassee, K., Chalker, S. L., Shaw, K. P., Ordidge, K. L., Badar, A., Janes, S. M., Blower, P. J., Lythgoe, M. F., Hailes, H. C., Tabor, A. B. (2013). Incorporation of paramagnetic, fluorescent and PET/SPECT contrast agents into liposomes for multimodal imaging. Biomaterials, 34, 1179 –1192. https://doi.org/10.1016/j.biomaterials.2012.09.070
We have developed lipopolyplexes formulated from a ternary mixture of lipids, peptides and DNA for the targeted delivery of pDNA to tumor cells. The peptide component includes a cationic domain to condense pDNA and a cancer cell-targeting sequence that is displayed at the surface of the nanoparticle. Mixtures of cationic and neutral lipids are used, including PEG or n-EG lipids for shielding, co-formulated with the neutral lipid DOPE. These powerful gene delivery vectors give transfection levels comparable with viral vectors. They can incorporate pDNA coding for biosensors or lethal genes, or can be tuned to deliver siRNA. In addition, we can add fluorophores for optical imaging, and co-deliver small molecule therapeutics.
Bofinger, R., Zaw-Thin, M., Mitchell, N. J., Patrick, P. S., Stowe, C., Gomez-Ramirez, A., Hailes, H. C., Kalber, T. L., Tabor, A. B. (2018) Development of lipopolyplexes for gene delivery: A comparison of the effects of differing modes of targeting peptide display on the structure and transfection activities of lipopolyplexes. J Pept Sci, 24, e3131. http://dx.doi.org/10.1002/psc.3131
Weitsman, G., Mitchell, N. J., Evans, R., Cheung, A., Kalber, T. L., Bofinger, R., Fruhwirth, G. O., Keppler, M., Wright, Z. V. F., Barber, P. R., Gordon, P., de Koning, T., Wulaningsih, W., Sander, K., Vojnovic, B., Ameer-Beg, S., Lythgoe, M., Arnold, J. N., Årstad, E., Festy, F., Hailes, H. C., Tabor, A. B., Ng, T. (2017) Detecting intratumoral heterogeneity of EGFR activity by liposome-based in vivo transfection of a fluorescent biosensor. Oncogene, 36, 3618 – 3628. https://doi.org/10.1038/onc.2016.522
Kudsiova, L., Welser, K., Campbell, F., Mohammadi, A., Dawson, N., Cui, L. L., Hailes, H. C., Lawrence, M. J., Tabor, A. B. (2016) Delivery of siRNA using ternary complexes containing branched cationic peptides: the role of peptide sequence, branching and targeting. Mol Biosyst, 12, 934 – 951. https://doi.org/10.1039/C5MB00754B
We also have expertise in bioconjugation to the surface of other nanoparticles, such as SPIONs, and are currently developing peptide-targeted semiconducting polymer nanoparticles (SPN) for photoacoustic imaging.
Thin, M. Z., Allan, H., Bofinger, R., Kostelec, T. D., Guillaume, S., Connell, J. J., Patrick, P. S., Hailes, H. C., Tabor, A. B., Lythgoe, M. F., Stuckey, D. J., Kalber, T. L. (2020) Multi-modal imaging probe for assessing the efficiency of stem cell delivery to orthotopic breast tumours. Nanoscale, 12, 16570. https://doi.org/10.1039/D0NR03237A
Stahl, T., Bofinger, R., Lam, I., Fallon, K. J., Johnson, P., Ogunlade, O., Vassileva, V., Pedley, R. B., Beard, P. C., Hailes, H. C., Bronstein, H., Tabor, A. B. (2017) Tunable Semiconducting Polymer Nanoparticles with INDT-Based Conjugated Polymers for Photoacoustic Molecular Imaging. Bioconj Chem, 28, 1734 – 1740. https://pubs.acs.org/doi/10.1021/acs.bioconjchem.7b00185
Current collaborative project
In collaboration with Dominique Bonnet and Ana Gomes (Francis Crick Institute), Helen Hailes and Vijay Chudasama (Chemistry, UCL). Funded by CRUK City of London Centre Development Fund.
In collaboration with Simon Walker-Samuel and Tammy Kalber (CABI, UCL) Rebecca Shipley (Mechanical Engineering, UCL), Marnix Jansen and Brian Davidson (Medical Sciences, UCL). Funded by a Cancer Research UK Multidisciplinary Award.
In collaboration with Ollie Ogunlade and Paul Beard (Medical Physics, UCL), Bob Schroeder (Chemistry, UCL), Tammy Kalber (CABI, UCL), Elnaz Yaghini (Medical Sciences, UCL)



