Investigating how immune cell neighbourhoods determine the epithelial phenotypic landscape and progression to cancer in Barrett’s oesophagus
Primary supervisor: Stuart McDonald, Queen Mary University of London
Secondary supervisor: Marco Novelli, UCL
Project
Barrett’s oesophagus (BO) is the only known precursor of oesophageal adenocarcinoma (EAC). It is characterised by the metaplastic replacement of the normal squamous epithelium of the distal oesophagus with a columnar epithelial phenotype as a result of chronic exposure to acid and bile reflux. BO can display a rich diversity of morphologically distinct glands associated with inflammation (1). We have recently published that these gland types represent an evolutionary process (2-4). Furthermore, we have shown that BO patients that progress to EAC often present with an increased diversity of gland phenotypes (Figure 1). We know little of the epithelial: immune cell relationship in determining BO gland phenotype nor its role in the progression to cancer.
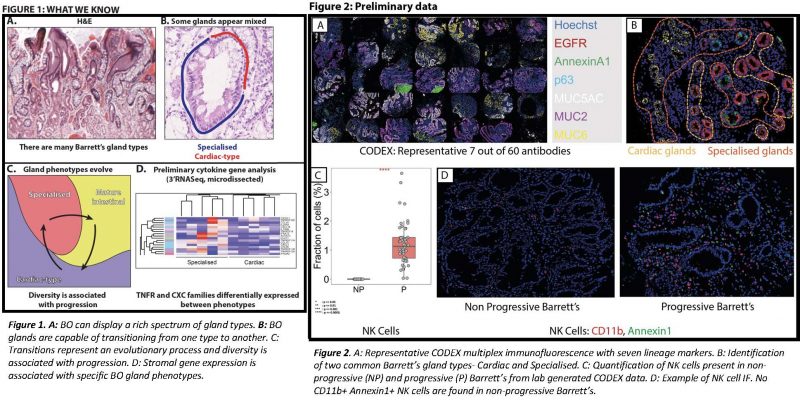
Preliminary data: Our lab has subjected tissue sections of progressive and non-progressive BO metaplasia to massive multiplex immunofluorescence (CODEX) (5) using a panel of 60 antibodies (Figure 2A) that label all epithelial (Figure 2B) and immune cells present in BO. The spatial arrangement of cells present in BO metaplasia has revealed the cell neighbourhood landscape is remarkably different in patients that show progressive metaplasia compared to non-progressive BO, to the extent that natural killer cells are exclusive to progressors (Figure 2C&D) and while specific neighbourhoods are gained (Tregs, Neutrophils), some are lost (plasma cells). Together, these data show unique epithelial and immune landscapes in progressive BO.
Hypothesis: That epithelial:immune cell crosstalk in BO is essential for progression to cancer
Aims: 1) To determine the immune cell neighbourhoods responsible for the generation of gland phenotype diversity. Using a large cohort of approximately 1000 biopsies from patients with either progressive or non-progressive BO, the immune neighbourhood surrounding each gland type will be investigated using targeted multiplex immunofluorescence guided by our CODEX data described above. 2) Longitudinal assessment of the epithelial:immune cell relationship will be investigated using a cohort of temporal biopsies over time from progressive and non-progressive BO metaplasia to determine predictive cellular biomarkers of cancer risk. 3) The effect of immune cells on epithelial phenotype will be tested in BO organoids – We have established a collaboration with Dr Matthew Stachler (Pathologist, UCSF, San Francisco) who will provide organoids established from progressive and non-progressive BO. Organoids will be exposed to flow sorted immune cells such as NK, Treg and neutrophils then immunophenotyped to determine the epithelial response to such stimulation. An established RNAseq protocol will determine major signaling pathways involved.
Candidate background
This project will suit a gastroenterologist or pathologist registrar with a longstanding interest in Barrett’s or the role of immune cells in the progression to cancer. The candidate will have a career-long demonstrable interest in clinical research. I am particularly interested in candidates with a drive to use bioinformatics as the basis of their research. The successful applicant will join a team that is part of Cancer Reseach UK’s grand challenge (STORMing Cancer) that is developing exciting methods to understand the stromal and immune cell contribution to carcinogenesis in Barrett’s.
References
- Schmidt et al., Evolutionary dynamics in Barrett oesophagus: implications for surveillance, risk, stratification and therapy. Nat Rev Gastroenterol Hepatol 19(2):95-111
- Evans et al. Clonal transitions and phenotypic evolution in Barrett’s oesophagus. Gastroenterology 2022;162(4):1197-1209
- McDonald et al., The Barrett’s gland in phenotypic space. Cell Mol Gastroenterol Hepatol 2014:1(1):41-5
- Lavery et al., Evolution of oesophageal adenocarcinoma from metaplastic columnar epithelium without goblet cells in Barrett’s oesophagus. Gut 2016;65(6):907-13
- Schurch et al., Coordinated cellular neighbourhoods orchestrate antitumoural immunity at the colorectal cancer invasive front. Cell 2020; 183(8):838
