Characterisation of metabolic heterogeneity and evolution in IDH-mutant AML in response to targeted therapies
Primary supervisor: Paolo Gallipoli, Queen Mary University of London
Secondary supervisor: Lynn Quek, King’s College London
Project
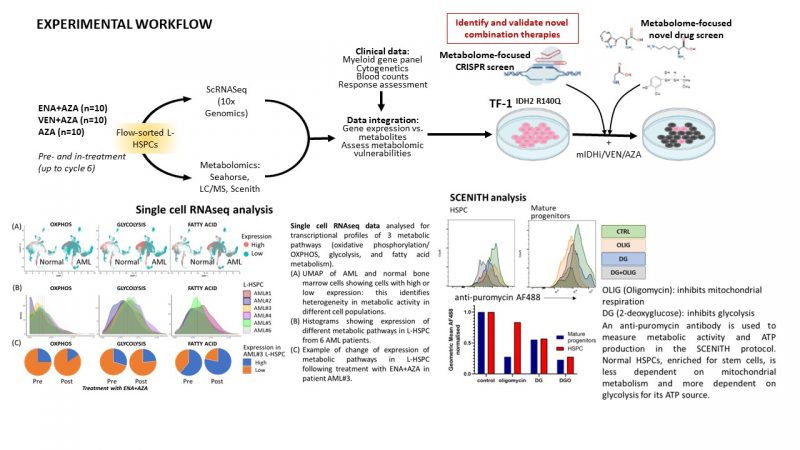
Acute myeloid leukaemia (AML) has a 5-year survival rates of only ~30%. Heterogeneity in mutations, gene expression and metabolic profiles are shaped by therapeutic pressures and drive resistance1-3. Leukaemic haematopoietic stem-progenitor cells (L-HSPC) have distinct metabolic features from normal HSPC to sustain their increased bioenergetic needs. However, we need to better understand how metabolism evolves in L-HSPC of patients undergoing treatment. AML mutated in isocitrate dehydrogenase (mIDH) 1 and 2 (~20% of AMLs) are an ideal model to study rewired metabolism. Enasidenib (ENA, a mIDH2 inhibitor) is approved to treat IDH-mutant AML, alone or in combination with hypomethylating agents (e.g. azacytidine, AZA)4. AZA is also combined with Venetoclax (VEN, a BCL2 inhibitor) to treat AML. With both regimens, although most patients respond, the majority then relapse. Furthermore, ~20-30% of patients are refractory to these treatments.
Through scRNAseq of L-HSPC from mIDH2 AML patients before and in-treatment with ENA+AZA (n=10) and AZA alone (n=5), we identified multiple distinct L-HSPC populations in individual patients. Consistent with recent independent evidence5, L-HSPCs express specific metabolic pathways at different stages of treatment, including upregulation of fatty acid (FA) metabolism following therapy. These indicate inter/intra-patient heterogeneity in L-HSPC phenotypes pre-treatment which evolve during therapy. We hypothesise that such metabolic states are drivers of leukaemogenesis which can be actioned therapeutically and yield biomarkers of response to current or future therapies.
Aims
1. Compare transcriptomic profiles of mIDH patients treated with ENA+AZA, VEN+AZA and AZA alone: obtain scRNAseq data on L-HSPC from mIDH patients, pre- and in-treatment with VEN+AZA (n=10), and a further 5 patients treated with AZA alone. Analyse these data with existing RNAseq data on patients treated with ENA+AZA.
Platforms: Illumina NGS, 10x Genomics Chromium – Quek group
2. Metabolomic profiling of L-HSPCs on longitudinal samples from mIDH2 patients treated with ENA+AZA (n=10) and VEN+AZA (n=10) compared with AZA alone (n=10). We will choose responders and non-responders patients to correlate findings with outcome.
Platforms: Seahorse metabolic flux analysis, Liquid Chromatography/Mass Spectrometry, SCENITH single cell flow cytometric metabolic profiling – Gallipoli group and collaboration with Mariia Yuneva and Richard Burt (Crick).
3. Integrate metabolomic data with RNAseq data to understand how changes to gene expression of metabolic genes relate to changes in relevant metabolites with validation of candidate pathways, such as FA metabolism, as actionable metabolic vulnerabilities following IDH-inhibitor therapy – Gallipoli and Quek groups.
4. Perform a metabolic-focused CRISPR/ drug screen in a mIDH2 AML cell line (TF-1IDH2R140Q) to assess synthetic lethality with ENA, AZA and VEN to provide orthogonal validation of pathways identified in Aims 1-3 and identify therapeutically actionable metabolic targets – both groups and collaboration with Prof JJ Schuringa, Groeningen, Netherlands.
Facilities and resources:
Equipment for flow cytometry cell sorting and metabolomic profiling, and expertise in analysis of RNAseq and metabolome data are established in the supervisors’ groups. Patient samples have been sourced from the AML-005 study (NCT02677922), and the Haematology Biobanks (Barts:REC#21/EE/0123, KCL:REC#18/NE/0141). We will use CoLC core facilities for Single Cell Genomics and the HPC Centre.
Candidate background
The innovative aspect of this project is the integration of longitudinal genomic and metabolomic data in patient AML samples to generate new insights in dysmetabolism which has potential for drug target discovery. There is a clear translational line of sight and is ideal for a clinician scientist interested in cancer multiomics.
References
- Quek, L. et al. Clonal heterogeneity of acute myeloid leukemia treated with the IDH2 inhibitor enasidenib. Nat. Med. 24, 1167-1177 (2018).
- Gallipoli, P. et al. Glutaminolysis is a metabolic dependency in FLT3ITD acute myeloid leukemia unmasked by FLT3 tyrosine kinase inhibition. Blood 131, 1639-1653 (2018).
- Woodley, K. et al. Mannose metabolism inhibition sensitizes acute myeloid leukemia to therapy by driving ferroptotic cell death. Nat. Commun. 14, 2132 (2023)
- DiNardo, C. D. et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. The lancet oncology 22, 1597-1608 (2021).
- Sirenko, M. et al. Deconvoluting clonal and cellular architecture in IDH-mutant acute myeloid leukemia. Abstract 1591 submitted to the 2023 American Society of Hematology Annual Scientific Meeting – https://ash.confex.com/ash/2023/webprogram/Paper186322.html
