Artificial intelligence and bioinformatics approaches to assess predictive biomarkers for immunotherapy and investigate optimum treatment combinations in metastatic breast cancer
Primary supervisor: Anthony Kong, King’s College London
Secondary supervisors: Teresa Marafioti, UCL, Heba Sailem, King’s College London
Project
A significant number of breast cancer patients develop brain metastasis (BM) or distant metastases, despite previous local and systemic treatments. For patients with oligometastasis, surgical excision or high dose radiotherapy such as radiosurgery (SRS) or stereotactic body radiotherapy (SBRT) to the metastases may help to achieve long-term local control. Following local treatments, patients will usually undergo further systemic treatments according to the breast cancer subtypes and previous treatments.
Patient-derived organoids (PDOs) have been shown to recapitulate patients’ tumours and could help to identify effective therapeutic regimens [1]. We have been generating PDOs from primary breast cancer tissues (Fig 1) [2]. We will be starting SOTO-BC study (IRAS 315793, chief PI: Dr. Kong), a prospective observation study to correlate the treatment Sensitivity of PDOs with Treatment Outcomes in breast cancer patients with brain or extra-cranial metastases. SOTO-BC study aims to generate PDOs from resected or biopsied brain and/or extra-cranial metastasis and assess the potential of these PDOs in predicting treatment outcome in patients.
Aim 1: To generate PDOs in SOTO-BC study and correlate the treatment sensitivity with patients’ outcomes
Breast cancer patients with limited brain or extra-cranial metastasis undergoing resection and/or biopsy will be recruited to SOTO-BC study and the samples will be used to generate PDOs together with collection of blood and translational research samples. The PhD candidate will test the recapitulation of genomic profiles and IHC markers of PDOs to that of human samples. The PDOs will be treated with the same treatments that the patients had previously received and will receive in order to correlate the treatment sensitivity with clinical outcome.
Aim 2: Assess genetic evolution and heterogeneity of primary tumours
To understand genetic drivers of metastasis, paired primary and metastatic samples from SOTO-BC will be subjected to exome- and RNA-sequencing. The candidate will work with a bioinformatician to assess how genetic profiles (mutations, copy number aberrations) evolved between primary tumours and brain/extracranial metastases and map the transcriptomic changes onto phylogenetic trees to unravel phenotype changes. They will work with the Dean Lab to identify patient-specific optimal therapies based on the sequencing data using biologically-informed neural network models trained on large in vitro and in vivo drug screening datasets (currently being developed in the lab).
Aim 3: Characterise the impact of identified driver mutations on tumour microenvironment phenotypes
The candidate will work with the Marafioti and Sailem groups to design imaging experiments to profile the tumour microenvironment phenotypes including various immune cancer cell types. They will utilise the Phenoimager HT multiplexed imaging system and CyTOF in these patients to correlate how the immune contexture changed with the genetic and phenotypic evolution. They will also determine how these phenotypes change in response to radiotherapy.
Aim 4: Validate optimum treatment (including immunotherapy) in PDOs
SOTO-BC study will have matched autologous PBMC from patients before and after treatments in addition to primary and recurrent samples. The candidate will validate optimum treatments from aims 2 and 3 in PDOs (+/- matched immune cells for immunotherapy).
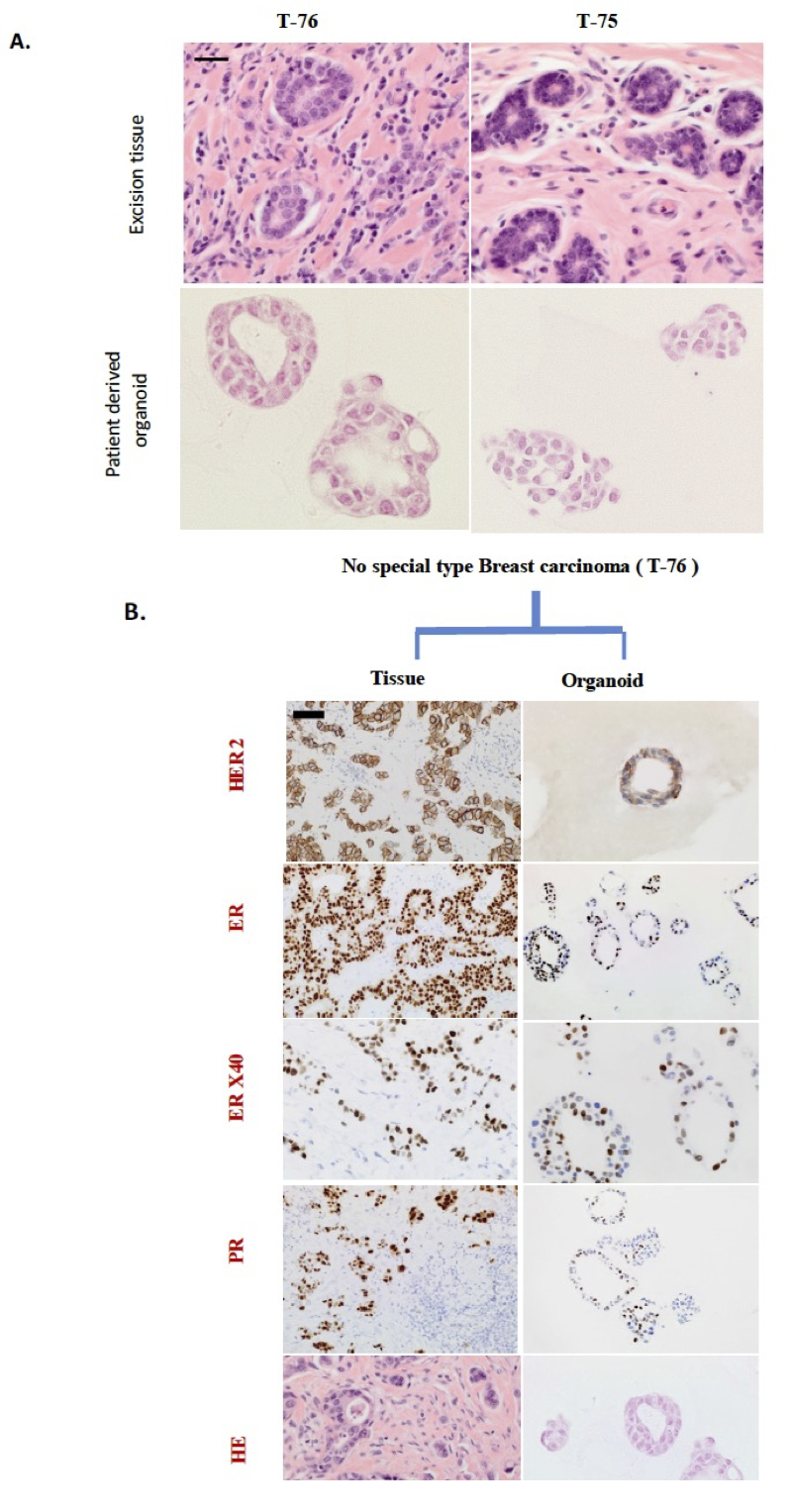
Figure 1: Histology and receptor status of excision tissue and corresponding patient-derived breast organoids
A. Breast cancer organoids architecture resembles their histology epithelium. H&E staining of tumour biopsy and its derived organoids have resembling tubular structures and organoids show the presence of two lumens recapitulating tumour features. The core biopsy of normal tissue (on right) displays tubules, and its corresponding organoid resembles mitotic cluster and absence of lumen. Scale bars = µ100 M
B. Comparative histological and immunohistochemical images of tumour tissue (left) and corresponding patient derived organoids (right). Shown are the representative example of no special type HER2 positive breast carcinoma. Tissue (left) presents with characteristics of tumour epithelium and surrounded by inflammatory cells, while organoids (right) recapitulate tumour epithelium (H&E). The excision tissue derived organoids recapitulate the expression of immunohistochemical markers; ER, PR, and HER2 status. The occasional ER and PR negative cells found in the HER2 positive excision tissue are retained in the derived organoids. Scale bars = µ100 M
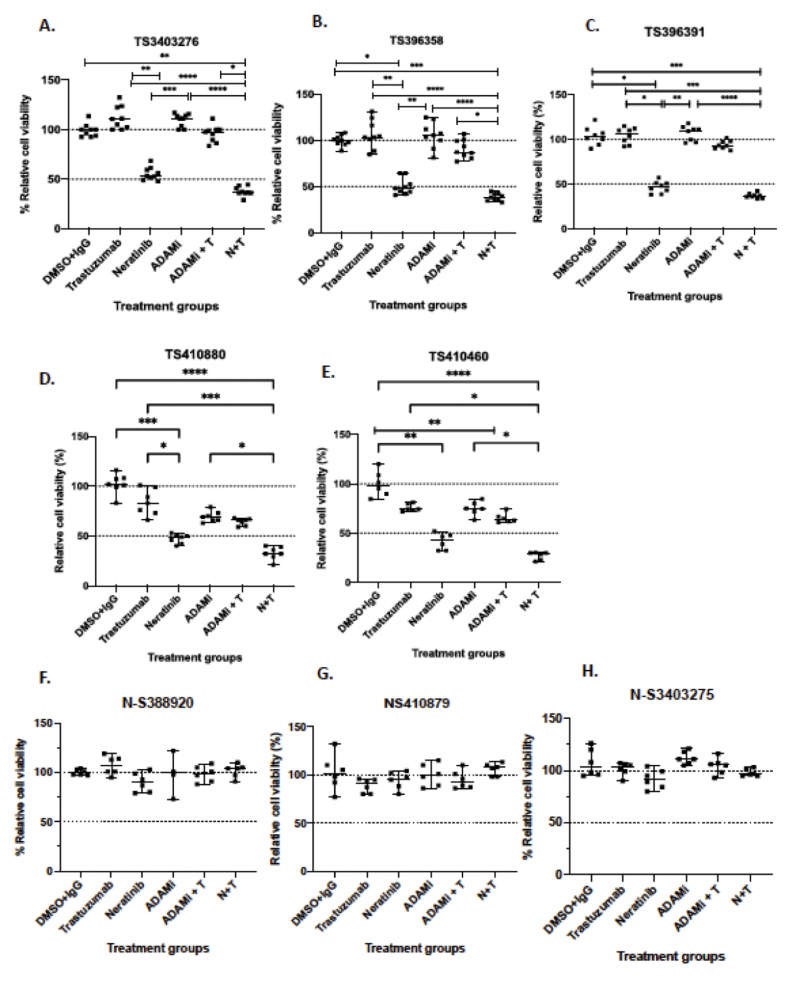
Figure 2: Combination of trastuzumab, neratinib, ADAM10/17 inhibitor INCB7839 or their combination in a panel of patient-derived organoids. A panel of breast cancer organoids some of their corresponding normal organoids were treated with neratinib (300 nM), trastuzumab (40ug/ml) and ADAM10/17 inhibitor INCB7839 (300nM) for 5 days and cell viability was assessed using 3D cell titer Glo and the results were normalized to control (DMSO + IgG). Data presented from 3 independent experiments (n=3) with 3 technical replicates; data is shown as the median ± range; non-parametric Kruskal Wallis test was performed p values are denoted as p* = 0.0332, ** p=0.0021, ***p=0.0002, ****p<0.0001.
Candidate background
This PhD programme would suit a specialist registrar trainee in medical or clinical oncology who is interested in pursuing a PhD in translational research using a multi-disciplinary approach with an aim to pursue an academic career. Ideally, the candidate has had previous laboratory experience through previous BSc and/or MSc projects but this is not essential as laboratory training will be provided.
References
- Sachs N et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 2018;172(1-2):373-386.e10.
- Arshad M, Azad A, Feldinger K, Vigneswara V, Nafi SNM, Santo CD, Zuo J, Shaaban A and Kong A. Neratinib could be effective as monotherapy or in combination with trastuzumab in HER2 lower-expressing breast cancer cells and organoid models. Manuscript in revision after peer review by British Journal of Cancer.
- Panayi C et al. Microenvironmental immune cell alterations across the spectrum of nodular lymphocyte predominant Hodgkin lymphoma and T-cell/histiocyte-rich large B-cell lymphoma. Front Oncol 2023 Oct 3:13:1267604. doi: 10.3389/fonc.2023.1267604. eCollection 2023.
- Dean J et al. Phase I study of a novel glioblastoma radiation therapy schedule exploiting cell-state plasticity. Neuro Oncol 2023;25(6):1100-1112.
- Sailem H et al. KCML: a machine-learning framework for inference of multi-scale gene functions from genetic perturbation screens. Mol Syst Biol. 2020;16(3):e9083.
