Uncovering the role of RNA regulator MYBBP1A in acute myeloid leukaemia
Primary supervisor: Diu Nguyen, Queen Mary University of London
Secondary supervisor: Eric So, King’s College London
Project
Acute myeloid leukaemia (AML) is an aggressive haematological malignancy with a 5-year survival rate of only ~30%. AML is propagated by leukaemia stem cells (LSCs), which can self-renew and evade chemotherapy and induce relapse [1]. Thus, to allow for more effective treatment of AML, it is critical to identify and target novel functional pathways regulating LSCs. RNA binding proteins (RBPs) have recently emerged as new critical regulators in stem cells. RBPs are central arbiters of post-transcriptional processes including RNA processing, degradation and translation. Mutations and aberrant expression of RBPs have been implicated in AML [2]. Importantly, the identification of dysregulated RBPs in leukaemia has led to a rapid development of RBP-directed therapeutic strategies [3].
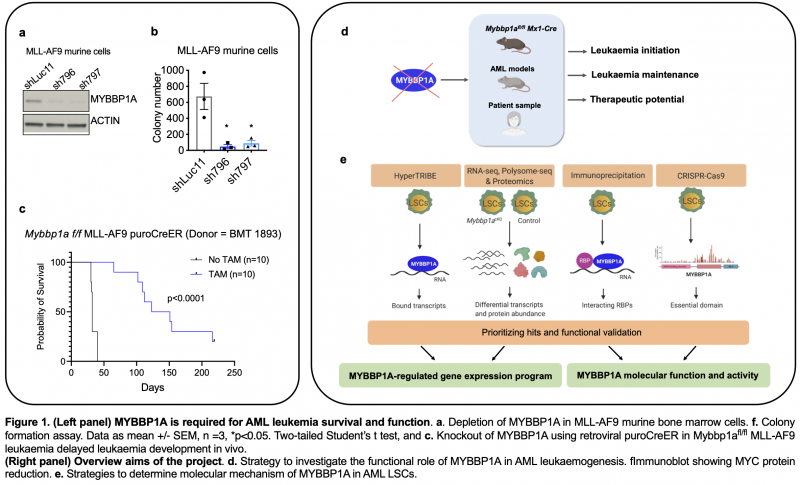
We initially identified a novel RNA binding protein MYBBP1A as one of the top hits in a screen for essential regulators of AML LSCs. Although MYBBP1A was reported to act as a co-transcription factor [4], we and others [5] have found MYBBP1A to bind several types of RNA in AML cell lines. Yet, its precise functions in RNA biology is not known and its role in malignant haematopoiesis is not understood. Our preliminary data show that MYBBP1A loss impaired clonogenicity of AML cells and delayed leukaemia development in murine MLL-AF9 driven leukaemia model (Figure 1). In this project, the candidate will combine the expertise and technologies from the host labs to test the main hypothesis that MYBBP1A drives survival and function of AML LSCs, via a post-transcriptional mechanism.
We have successfully developed a Mybbp1a conditional knockout mouse model Mybbp1afl/fl Mx1-Cre. Combining this novel tool with MLL-AF9 and HOXA9/MEIS1 driven AML models, we will address the following aims (Figure 1).
Aim 1: Investigating the role of MYBBP1A in AML leukaemogenesis. We will assess how Mybbp1a loss affects leukaemia initiation and maintenance, by inducing Mybbp1a deletion before or after leukaemia transformation of haematopoietic stem/progenitor cells, respectively. The functional requirement of Mybbp1a in LSCs will be examined using serial colony replating, serial transplantation and limiting-dilution transplantation. Moreover, we will determine MYBBP1A therapeutic potential, using human AML primary cells, reconstructed models and xenografts and in combination with chemotherapies and Venetoclax.
Aim 2: Elucidating molecular basis for MYBBP1A function in leukaemic stem cells. To achieve this aim, we will map MYBBP1A-bound transcripts using HyperTRIBE or iCLIP approach. We will identify the gene expression programmes controlled by MYBBP1A using integrative analyses at multiple regulatory levels including transcriptome, translatome and proteome in LSCs. Since MYBBP1A has also been known to play a role in rRNA biosynthesis, we will interrogate the effects of Mybbp1a loss on ribosome biogenesis and translation regulation in LSCs. Overall, this study will uncover the functional relevance, hence therapeutic potential of MYBBP1A in AML, and reveal the underlying molecular mechanism for MYBBP1A’s role in LSCs.
Candidate background
This project has a clear translational line of sight and is ideal for a clinician scientist interested in cancer and/or RNA biology. The ideal candidate will have a strong drive to answer fundamental research questions and desire to pursue a scientific career or to combine with their clinician career.
References
- Lapidot T., et al. 1994. Nature.
- Nguyen D., et al. 2020. Nat. Commun.
- Prieto C., et al. 2020. CSHL perspective in medicine
- Felipe-Abrio B., et al. 2020. Cancers (Basel)
- Vu L., et al. 2017a. Nat. Gen.
