Impact of co-occurring del(1p)/gain(1q) events in the evolution of multiple myeloma
Primary supervisor: Kwee Yong, UCL
Secondary supervisor: Dinis Calado, Francis Crick Institute
Project
Background: Myeloma, the second most common form of blood cancer, poses a significant challenge as it increases in incidence whilst remaining incurable [1]. It is preceded by an asymptomatic precursor phase called smouldering myeloma. Currently, our understanding of myeloma revolves around a model whereby a B-cell undergoes an initiating genetic event and progresses through the different disease phases by acquiring secondary events. Events offering a competitive advantage are extramedullary, and treatment-resistant forms of the disease [2]. The acquisition of secondary events affecting both the short and long arm of chromosome 1 together is common in these settings, and increases both during disease progression and relapse [3-4]. However, the functional impact of these double-hit, arm-level events, remains largely unstudied hindering our understanding and management of these cases.
Hypothesis: We hypothesise that the co-occurrence of del(1p) and gain(1q) events drive an aggressive disease phenotype leading to disease evolution and treatment resistance that can be targeted therapeutically.
Aims and Objectives (Figure 1):
To test this hypothesis, the aims of this project are twofold:
- Aim 1: Assessing the impact of the co-occurrence of del(1p)/gain(1q) in vitro. Using luciferase-positive myeloma cell lines with a normal chromosome 1, we will induce a del(1p) and gain(1q) using Karyocreate. 5 Wild-type and co-deleted cells will be sorted and assessed for survival, proliferation, and colony formation as previously described. Using RNA sequencing and PerturbSci-Kinetics we will assess differential expression and RNA kinetics. Finally, we will assess drug response to standard anti-myeloma agents. This aim will determine the molecular and biological consequences of double-hit del(1p)/gain(1q) and give mechanistic insight into this aggressive disease phenotype.
- Aim 2: Assessing the impact of the co-occurrence of del(1p)/gain(1q) in vivo. Using the previously established luciferase-positive cell lines we will perform intra-tibial injections of either 100% wild type, 100% del(1p)/gain(1q) or subclonal del(1p)/gain(1q) populations in NSG mice. We will assess mice for 1) tumour growth 2) extramedullary disease formation and 3) survival. Moreover, we will assess at the single-cell level 1) the clonal composition and evolution of the marrow and extramedullary sites and 2) the impact of these double-hit del(1p)/gain(1q) cells on the bone-marrow stroma. This work will identify the impact of this double-hit event on 1) disease phenotype, 2) clonal evolution 3) the non-immune microenvironment.
Results will shed light on the functional consequences of del(1p)/gain(1q) alterations, providing insights into the mechanisms driving progression from precursors to relapsed and refractory disease, paving the way for targeted therapeutic interventions in myeloma and potentially other cancers featuring similar chromosomal events.
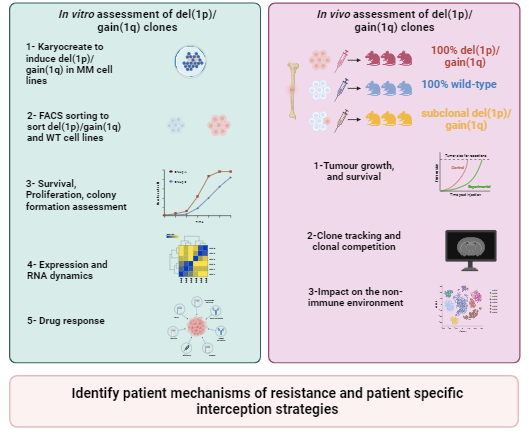
Figure 1: Summary of the project. To test the hypothesis that double-hit del(1p)/gain(1q) events drive an aggressive disease phenotype leading to treatment resistance that can be targeted therapeutically we will assess the impact of this event both in vitro and in vivo. Aim 1: using luciferase-positive myeloma cell lines with a normal chromosome 1 (e.g. KMS12BM, KMS34, INA6, AMO1), we will induce a del(1p) and gain(1q) using Karyocreate. Wildtype and co-deleted cells will be sorted by flow cytometry and deletions confirmed by low-pass whole genome sequencing and interphasic fluorescence in situ hybridisation. Single hit cells (del(1p) or gain(1q)) will be stored for another analysis. We will assess survival, proliferation, and colony formation as previously described. Using RNA sequencing and PerturbSci-Kinetics we will assess differential expression and RNA kinetics. Finally, we will assess drug response to standard anti-myeloma agents. This aim will determine the impact of double-hit del(1p)/gain(1q) events on myeloma survival, progression, and proliferation and give some mechanistic insight into this aggressive disease phenotype. Aim 2: Using the previously established luciferase-positive cell lines we will perform intra-tibial injections of either 100% wild type, 100% del(1p)/gain(1q) or subclonal del(1p)/gain(1q) populations in immunodeficient NSG mice. We will assess mice for 1) tumour growth 2) extramedullary disease formation and 3) survival. Moreover, using a PARSE-seq platform, we will assess at the single cell level 1) the clonal composition and evolution of the marrow and extramedullary sites and 2) the impact of these double-hit del(1p)/gain(1q) cells on the bone marrow stroma cells by sequencing live bone marrow cells (Dapi-/Calcein+), depleted for cells expressing erythroid markers (CD71/Ter119) and immune lineage markers (CD45/CD3/B220/CD19/Gr1/CD11b) as previously described. This work will identify the impact of this double-hit event on 1) extramedullary disease 2) clonal evolution 3) the non immune microenvironment and help us understand disease progression. Diagram performed using BiorenderTM.
Candidate background
This project will provide an all-round training in genomics of cancer evolution, employing advanced technologies including CRISPR engineering, mouse models and bioinformatics. Applications are encouraged from highly motivated individuals with an interest in translational research aimed at benefiting haematology patients. Ideal candidates should possess a medical background with a special interest in haematology, and especially haematology trainees with an interest in cancer evolution and genetics. Under-represented minority candidates are encouraged to apply.
References
- Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016;43(6):676-681.
- Boyle EM, Davies FE. From little subclones grow mighty oaks. Nat Rev Clin Oncol 2023; 20: 141-142.
- Boyle EM*, Blaney P*, Stoeckle JH*, Wang Y, Ghamlouch H, Choi J, Braunstein M, Kaminetzsky D, Montes L, Corre J, et al Multiomic Mapping of Somatic Copy Number Abnormalities and Structural Variation to the Chromatin Structure of Chromosome 1 Highlights Multiple Recurrent Regions and Novel Disease Drivers, Clin Cancer Res Off J Am Assoc Cancer Res 2023, in press
- Boyle EM, Deshpande S, Ashby C, Tytarenko RG, Wang H, Wang Y et al. The Molecular Make Up of Smoldering Myeloma Highlights the Evolutionary Pathways Leading to Multiple Myeloma. Nat Commun 12, 293 (2021)
- Bosco N, Goldberg A, Zhao X, et al. KaryoCreate: A CRISPR-based technology to study chromosome-specific aneuploidy by targeting human centromeres. Cell. 2023;186(9):1985-2001.e19.
